
What Is Qelbree?
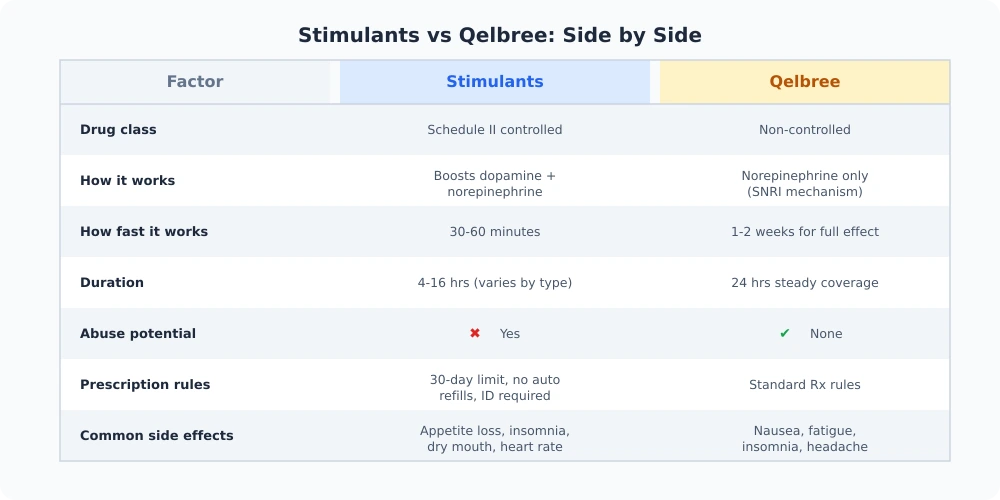
Qelbree (viloxazine extended-release capsules) is a non-stimulant medication approved by the FDA for the treatment of ADHD in children aged 6 and older, adolescents, and adults. It received pediatric approval in April 2021 and expanded to adult use in April 2022, making it the first novel non-stimulant option for adults with ADHD in over 20 years.
Unlike stimulant medications such as Adderall or Vyvanse, Qelbree is not a controlled substance. It carries no DEA scheduling, no abuse potential documented in clinical trials, and no prescription refill restrictions tied to controlled substance regulations. For patients and providers, that translates to simpler prescribing, easier pharmacy pickups, and fewer logistical barriers to consistent treatment.
If you've struggled with stimulant side effects, have a history that makes controlled substances a concern, or simply want to understand all available options before starting treatment, Qelbree deserves a serious look.
How Viloxazine Works in the Brain
Qelbree is classified as a selective norepinephrine reuptake inhibitor (NRI). It blocks the norepinephrine transporter, increasing norepinephrine levels in the prefrontal cortex, amygdala, and nucleus accumbens--brain regions directly involved in attention, impulse control, and emotional regulation.
But viloxazine does more than just boost norepinephrine. Research shows it also moderately increases dopamine in the prefrontal cortex through a secondary mechanism: because the norepinephrine transporter also handles dopamine reuptake in that region, blocking it raises both neurotransmitters where they matter most for ADHD symptoms.
There's also evidence that viloxazine modulates serotonin activity, which may contribute to its effects on mood and emotional regulation. This dual-pathway action distinguishes it from older non-stimulants like atomoxetine (Strattera), which primarily targets norepinephrine alone.
The clinical result: improved focus, reduced impulsivity, and better emotional control--without the dopamine surge in reward pathways that gives stimulants their abuse potential. For a broader picture of how ADHD medications compare across classes, see our full medication comparison.
Who Should Consider Qelbree
Qelbree isn't for everyone, and it isn't trying to replace stimulants wholesale. Current evidence suggests stimulants remain more effective at symptom reduction for most patients. But there are specific clinical scenarios where Qelbree becomes the right first choice--or a strong second option:
Patients with substance use history. Because Qelbree has no abuse potential and isn't a controlled substance, it's often preferred for patients in recovery or those with a history of stimulant misuse. There's no risk of diversion and no DEA-related prescribing friction.
People who can't tolerate stimulants. Stimulant side effects like insomnia, appetite suppression, elevated heart rate, or anxiety worsen for some patients to the point where the medication creates as many problems as it solves. Qelbree's side effect profile is different enough that patients who fail stimulant trials sometimes respond well.
Co-occurring anxiety. Stimulants can amplify anxiety in patients with comorbid anxiety disorders. Qelbree's serotonin modulation may offer a gentler profile for these patients, though individual responses vary.
Patients who need consistent 24-hour coverage. Stimulants have an on/off quality--they peak and fade within hours. Qelbree provides steady, once-daily coverage without the afternoon crash or the morning wait for onset. For people whose ADHD disrupts sleep routines, evening functioning, or early mornings, this consistency matters.
Children and teens. Parents understandably have concerns about controlled substances. Qelbree gives families a clinically validated option that doesn't carry the same regulatory or stigma-related baggage. It's approved for children as young as 6.
Dosing: What to Expect
Qelbree is taken once daily, in the morning, with or without food. The capsules come in 100 mg, 150 mg, and 200 mg strengths and can be opened and sprinkled on applesauce for patients who have difficulty swallowing pills--a practical advantage for younger children.
Children (ages 6--11): Starting dose is 100 mg once daily. Your provider may increase by 100 mg weekly up to a maximum of 400 mg per day, depending on response and tolerability.
Adolescents (ages 12--17): Starting dose is 200 mg once daily, titrated up to 400 mg per day if needed.
Adults (18+): Starting dose is 200 mg once daily, with gradual increases of 200 mg per week up to a maximum of 600 mg per day.
Unlike stimulants, which produce noticeable effects within 30 to 60 minutes, Qelbree takes longer to reach full therapeutic effect. Most patients begin noticing improvement within one to two weeks, though some may need up to four weeks. This is standard for non-stimulant medications and requires patience during the titration period.
Side Effects and Safety
Like all medications, Qelbree has side effects. The good news: most are mild to moderate and tend to diminish within the first few weeks of treatment. The most commonly reported side effects differ slightly between age groups.
In children and adolescents (ages 6--17): The most common side effects include drowsiness, decreased appetite, fatigue, nausea, vomiting, trouble sleeping, and irritability.
In adults (18+): The most common side effects include insomnia, headache, drowsiness, fatigue, nausea, decreased appetite, dry mouth, and constipation.
Cardiovascular monitoring: Clinical trial data showed that between 22% and 31% of pediatric patients experienced increases in heart rate of at least 20 beats per minute. Providers should assess heart rate and blood pressure before starting treatment, at dose increases, and periodically during therapy.
Boxed warning -- suicidal ideation: Qelbree carries an FDA boxed warning about the potential for increased suicidal thoughts and behaviors, particularly in the first few months of treatment or during dose changes. This warning applies to all patients, children and adults alike. Providers should screen for personal and family history of suicidal ideation before prescribing, and patients should report any changes in mood or behavior immediately.
This boxed warning is shared by other medications in the same pharmacological class and reflects the FDA's standard approach to drugs that affect norepinephrine and serotonin pathways. It does not mean Qelbree commonly causes suicidal behavior--it means close monitoring is warranted, which any responsible provider will build into your care plan.
Qelbree vs. Strattera (Atomoxetine)
The most natural comparison for Qelbree is Strattera, since both are non-stimulant NRIs. But there are meaningful differences:
Onset of action. Qelbree typically reaches full effectiveness in one to two weeks. Strattera is slower, often requiring four to six weeks before patients see the full benefit. For patients who've been waiting months to get treated, those extra weeks matter.
Mechanism nuance. While both inhibit norepinephrine reuptake, Qelbree also modulates serotonin activity. This additional pathway may contribute to better mood regulation in some patients, though head-to-head trial data directly comparing the two medications in the same population is limited.
Side effect profile. Strattera is more commonly associated with nausea, dry mouth, and mood changes, particularly in the early weeks. Qelbree's side effect profile, while overlapping, tends to favor drowsiness and fatigue over gastrointestinal issues. Individual variation is significant, though, so what one patient tolerates poorly another may handle fine.
Approval timeline. Strattera has been on the market since 2002 and carries a deeper evidence base. Qelbree, approved in 2021 for pediatric use and 2022 for adults, is newer. That means less long-term outcome data, but it also means it reflects more recent pharmacological understanding of ADHD.
Qelbree vs. Guanfacine (Intuniv)
Guanfacine works through a completely different mechanism--it's an alpha-2 adrenergic agonist that calms the overactive stress response rather than targeting norepinephrine reuptake. It's particularly helpful for hyperactivity and emotional dysregulation, and it's often combined with a stimulant rather than used alone.
Qelbree, by contrast, is designed as a standalone ADHD treatment. Its mechanism targets the core attention and impulse-control pathways more directly. If your primary symptoms are inattention and executive dysfunction rather than hyperactivity and emotional reactivity, Qelbree may be the more targeted option.
Guanfacine also carries a notable side effect of low blood pressure and sedation, which limits its utility for some patients. Qelbree's cardiovascular profile is different--heart rate increases are more common than blood pressure drops.
What the Clinical Trials Show
Qelbree's FDA approval was based on clinical trial data from 1,289 participants across 59 sites in the United States. The trials included two studies in children aged 6 to 11 and one in adolescents aged 12 to 17, all randomized, double-blind, and placebo-controlled. A separate phase 3 trial supported the adult indication.
In the pediatric trials, patients treated with Qelbree showed statistically significant reductions in ADHD symptoms compared to placebo, as measured by the ADHD Rating Scale (ADHD-RS-5). Improvement was observed as early as week one in children at the 100 mg and 200 mg dose levels, and by week two in adolescents at 400 mg.
In the adult trial, patients on flexible doses of 200 to 600 mg achieved the primary endpoint of reduced symptom scores on the Adult ADHD Investigator Symptom Rating Scale (AISRS). The key secondary endpoint--improvement on the Clinical Global Impression--Severity scale--was also met with statistical significance.
It's worth noting that current evidence indicates viloxazine produces moderate symptom reductions, roughly comparable to atomoxetine but generally less than stimulants like methylphenidate. For patients where stimulants are the right fit, they remain the most effective pharmacological option. Qelbree fills a different need--it's the better choice when stimulants aren't appropriate, not a replacement for them across the board.
Getting Started with Qelbree at ADHD One
If you think Qelbree might be right for you or your child, the first step is a comprehensive ADHD evaluation. Your provider needs a clear diagnostic picture before recommending any medication, and that includes screening for comorbidities that could influence which drug class makes the most sense.
At ADHD One, you can schedule an evaluation the same day you decide to pursue treatment. If Qelbree is clinically appropriate, your provider will discuss the titration plan, expected timeline to full effect, and what to monitor during the first few weeks. Because Qelbree isn't a controlled substance, the prescription process and ongoing refill check-ins are more streamlined than with stimulant medications.
Already know you want to explore your options? Begin treatment within a single appointment. If your provider determines that a stimulant is the better starting point, you can read about how Adderall prescribing works at ADHD One to understand that pathway as well.
Frequently Asked Questions
Is Qelbree a controlled substance?
No. Qelbree (viloxazine) is not classified as a controlled substance by the DEA. Clinical studies showed no evidence of abuse potential. This means prescriptions are easier to fill, there are no quantity limits per refill, and providers can prescribe with fewer regulatory restrictions than stimulants like Adderall or Vyvanse.
How long does it take for Qelbree to start working?
Most patients begin noticing symptom improvement within one to two weeks. Some clinical trial data showed improvement as early as week one at certain doses. However, full helpful effect may take up to four weeks, and your provider may adjust your dose during this period. Unlike stimulants, which work within an hour, non-stimulants require a gradual build-up in your system.
Can Qelbree be taken with stimulant medications?
Combining Qelbree with stimulants is not standard practice and was not studied in the clinical trials that led to its approval. However, combination strategies are sometimes considered in treatment-resistant cases. This is a decision your provider would make based on your specific clinical profile, tolerability, and treatment history. Never combine medications without medical supervision.
Does Qelbree cause weight loss?
Decreased appetite is a commonly reported side effect, which may lead to weight changes in some patients. In clinical trials, pediatric patients were monitored for weight changes, and providers are advised to track weight during treatment. The appetite effects tend to be less pronounced than those seen with stimulant medications, but individual responses vary. Discuss any concerns about weight with your provider.
What if Qelbree doesn't work for me?
Not every medication works for every patient, and that's expected in ADHD treatment. If Qelbree doesn't provide adequate symptom control after a full dose adjustment trial, your provider may recommend switching to a different non-stimulant, trying a stimulant medication, or adjusting your overall treatment plan. ADHD management is iterative--the goal is finding the right fit, not committing to the first option forever.
Related ADHD Guides
Had bariatric surgery? Non-stimulant medications like Qelbree may absorb differently after weight loss procedures. Read our guide to ADHD medication after gastric bypass for important absorption considerations.